Absolute Temperature of Ideal Gas Is Directly Proportional to
B the average momentum of a molecule of the gas. Using the ideal gas equation PV nRT.

Stranerd En Instagram Need Help With General Chemistry Send Us A Dm Today Dalton S Law Boyle S Law Avogadro S Law
In order for two quantities to be directly proportional you need one to increase as the other increases or decrease as the other decreases in both cases at the same rate.

. From ideal gas equation PV Nk B T 2 3 E. Expression for rms velocity. Charles law states that.
Where V Volume of a gas sample. Quite simply put it says that Gases expand on heating and contract on cooling. PV 2 3 E.
Explain how the volume and moles of gas were held constant during the second part of this experiment. Physics questions and answers. In other words the product of pressure and volume is constant for a fixed mass of ideal gas at fixed temperature.
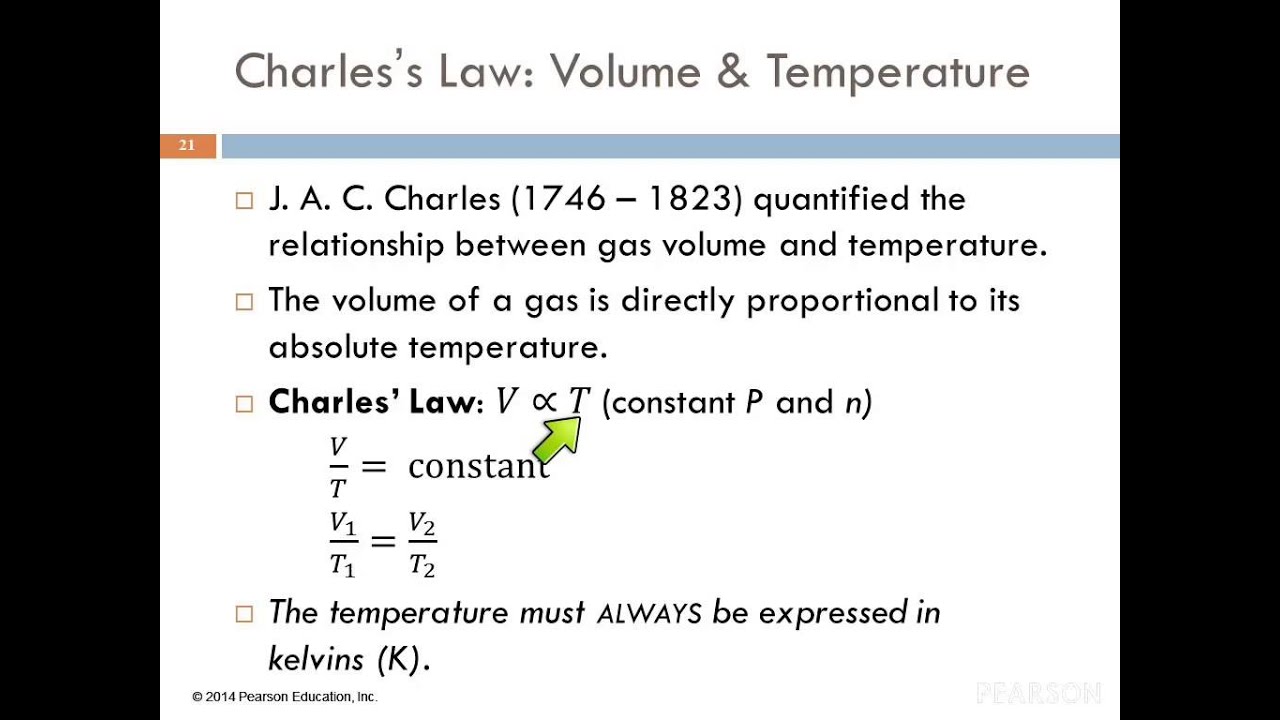
If the absolute temperature is double the volume is doubled. V nRT Nm v 2 3 nRT Nm. The volume of a gas is directly proportional to its absolute temperature.
Charles law states that When the pressure of a sample of air is held constant then the volume of the gas is directly proportional to its temperature that is. The average speed of its molecules the average momentum of its molecules the average kinetic energy of its molecules the mass of its molecules It is. Asked Sep 27 2016 in Physics Space Science by Bobby introductory-physics.
The relative increase In volume of the gas for temperature increase of 1 C the number of molecules In the sample the average translatianal kinetlc energy of the gas the avcragc momentum of a molecule of the gas the amount of heat requlred to raise the. At constant pressure the volume of fixed mass of gas is directly proportional to the Kelvin temperature of absolute temperature. The absolute temperature of an ideal gas is directly proportional to which of the following.
The gas volume is directly proportional to the gas temperature for a given fixed amount of gas kept at a constant pressure ie. This is Charles Law. Begin array lfrac NRT N_ Afrac 2NE 3end array.
The average kinetic energy of a collection of gas particles is directly proportional to absolute temperature only. QUESTION 18 The absolute temperature of an ideal gas is directly proportional to which one of the following quantities. Momentum Speed Kinetic energy Masss None of above Get the answer to your homework problem.
This is generally the case that as temperature increases density decreases but there is one exception to this which is water. 2 E 3 2 Nk B T. Boyles Law FormulaBoyles Law an ideal gas law which states that the volume of an ideal gas is inversely proportional to its absolute pressure at a constant temperature.
The volume V of a given mass of a gas at constant pressure P is directly proportional to its. But n N N A N N A. Charless law or the law of volumes was found in 1787 by Jacques Charles.
The absolute temperature of an ideal gas is directly proportional to a the number of molecules in the sample. V volume of one mole of the gas. V T where P and n are constant or V kT where k is a constant.
Kinetic Molecular Theory states that gas particles are in constant motion and exhibit perfectly elastic collisions. Let P pressure exerted by one mole of an ideal gas. Pressure exerted by gas is given by P 31.
PV nRT implies P nRTV Since R represents a constant you can write it separately. For a given mass of an ideal gas the volume and amount moles of the gas are directly proportional if the temperature and pressure are constant. Hence the correct answer is option B.
At constant temperature the relation between the volume and temperature of a quantity of gas can be written as V T. The average value of the pressure of the gas is P N V m v 1 3 N V m v 2. V propto T.
Mathematically charles law can be expressed as. It states that for a given mass of an ideal gas at constant pressure the volume is directly proportional to its absolute temperature assuming in a closed systemThe statement of Charless law is as follows. Also frac13fracMNC2 fracRNTkT Or frac12mC2frac32 kT or frac12mC2alpha T Where 32 k.
Thus the mean square velocity of the molecule will be v PV Nm v 2 3 PV Nm. A law that states that at constant temperature and pressure the volume of a sample of gas is directly proportional to the number of moles of gas in the sample. The absolute temperature of an ideal gas is directly proportional to which of the following quantities.
A law that states that at constant pressure the volume of a fixed amount of gas is directly proportional to its absolute temperature in kelvins. V T Constant. Clearly we can see it is directly proportional to its pressure and inversely proportional to its absolute temperature.
V NRT N A Nm v 2 3 NRT N A Nm. The absolute temperature of an ideal gas is directly proportional to which of the following quantities. The absolute temperature of an ideal gas is directly proportional to which of the following average quantity of molecules.
Mathematically T α V or it can also be expressed as V 1 T 1 V 2 T 2 The fraction of T and VTV is always constant if the absolute pressure and mass of gas are constant. Charless law states that pressure and absolute temperature of an ideal gas are directly proportional when the volume and moles n of gas are constant. What is the relationship between volume and moles.
3 From equation 3 it can be concluded that the average energy of the molecules of a gas is directly proportional to the absolute temperature of the gas. C the average translational kinetic energy of the gas. Mass The answer is c.
Where T is the Temperature. More specifically for a fixed mass of gas at a constant pressure the volume V is directly proportional to the absolute temperature T. A plot of the effect of temperature on the volume of a gas at constant pressure shows that the volume of a gas is directly proportional to the number of moles of.
We can say that the square root of absolute temperature T of an ideal gas is directly proportional to the root mean square velocity of its molecules. The following is the deduction of kinetic theory in terms of pressure. D the diffusion constant of the gas.
Starting from the ideal gas law equation isolate P on one side of the equation first. If the absolute pressure and mass of gas are constantfixed the temperature of a gas is directly proportional to the volume occupied by the gas. Where M mass of one mole molecular weight of the gas.
Pin By James Kayawe On Chemistry Charles Law Chemistry Charles

Three Gas Laws Doodle Notes Science Doodle Notes Doodle Notes Doodle Notes Science Science Doodles

Pin Na Doske Chemistry Assignment

Javi On Twitter Boyle S Law Charles Law Ideal Gas Law

Unify Physics Unifyphysics Follow Unifyphysics Charles Law In 2021 Charles Law Physics Law

Charles S Law Definition Formula Examples Charles Law Teaching Place Values Law

Comments
Post a Comment